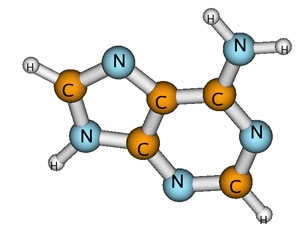
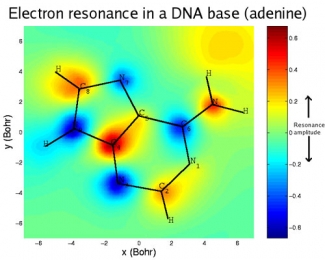
High-energy radiation is notorious for damaging DNA, primarily by breaking chemical bonds. Damage to DNA can cause mutations, cancer, or even death. Much of this damage is inflicted by secondary, or low-energy, electrons knocked out of atoms in the DNA molecules by radiation. The low-energy electrons get captured by the DNA bases (which make up the letters of the genetic code), temporarily forming a negatively charged molecule (anion). The anion lasts just long enough to transfer its excess energy to the weakest nearby chemical bond, often breaking it.
In DNA, the weakest link is the carbon-oxygen bond between the sugar and phosphate groups that form its backbone. Low-energy electrons can cause breaks in one or both strands of DNA. Although the letters of the code remain undamaged, these structural breaks increase the likelihood that the code will be misread and permanent, and potentially catastrophic, biological changes will ensue.